Gubra
375,00 DKK
-5,26 %
Mindre end 1K følgere
GUBRA
NASDAQ Copenhagen
Biotechnology & Pharmaceuticals
Health Care
Oversigt
Finansielt overblik og estimater
Ejerskab
Dividend
Investorkonsensus
-5,26 %
-8,94 %
-27,54 %
-22,20 %
-5,02 %
-30,30 %
-
-
+240,91 %
Gubra er en medicinalvirksomhed. Virksomhedens aktiviteter er fokuseret på de tidlige stadier af lægemiddeludvikling. De udfører hovedsageligt forskning og udvikling inden for stofskifte- og fibrotiske sygdomme.Virksomhedens produktportefølje omfatter flere brands og lægemidler, og aktiviteterne foregår på globalt plan med den største tilstedeværelse i Nordamerika og Norden. Hovedkontoret ligger i Hørsholm, Danmark.
Læs mereMarkedsværdi
6,13 mia. DKK
Aktieomsætning
23,42 mio. DKK
Omsætning
2,64 mia.
EBIT %
81,54 %
P/E
3,61
Udbytteafkast, %
-
Seneste analyse
Seneste analyse
Udgivelse: 01.12.2025
Omsætning og EBIT-margin
Omsætning mia.
EBIT-% (adj.)
EPS og udbytte
EPS (adj.)
Udbytte %
Alle
Analyse
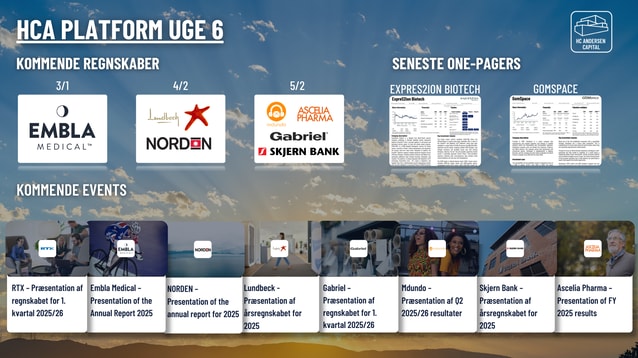
Selskabspræsentationer
Selskabsmeddelelser
ViserAlle indholdstyper
NOTICE TO CONVENE ANNUAL GENERAL MEETING 2026 GUBRA A/S
Gubra - Præsentation af årsregnskabet for 2025
Bliv en del af Inderes community
Gå ikke glip af noget - opret en konto og få alle de mulige fordele
Inderes konto
Følgere og notifikationer om fulgte virksomheder
Analytikerkommentarer og anbefalinger
Værktøj til at sammenligne aktier og andre populære værktøjer